
Advances in cancer diagnosis and treatment have raised five-year survival rates for all cancers nationwide from 49% in the mid-1970s to 69% in the 2010s. WashU Medicine scientists have been at the forefront of the national trend by continually expanding what physicians know about cancer and how to detect and treat it. Cutting-edge research at WashU Medicine has improved early detection of cancer, led to new, more potent therapies with fewer side effects, and personalized treatment for patients, with the aim of making therapies as effective as possible.
Here are just five examples of emerging developments from WashU Medicine physicians and researchers, with potential for changing the lives of cancer patients.
Clinical trial network aims to improve cancer screening in diverse populations
WashU Medicine is one of seven academic centers in a new clinical trials network launched by the National Cancer Institute (NCI) in 2024 to investigate emerging technologies for cancer screening, with the goal of reducing cancer-related illnesses and deaths.
WashU Medicine investigators work closely with Siteman Cancer Center to lead trials that take place in Missouri and southern Illinois. The network also aims to reach diverse populations, including people living in underserved areas.
A major focus of the network is to evaluate the effectiveness of a screening technology designed to detect multiple cancers with a single blood test. Some such multicancer detection tests already are in use, but they lack full FDA approval and continue to be under evaluation. The network will evaluate these tests, with the aim of helping fill a gap in early detection. Many cancers, such as pancreatic, lung, kidney, ovarian and liver tumors, can’t be identified through standard screening tests and often aren’t detected until they are too advanced to treat effectively.
“Multicancer detection tests could open the door to screening for many different types of cancer simultaneously,” said Aimee James, PhD, a WashU Medicine professor of surgery in the surgery department’s Public Health Sciences Division and co-leader of the prevention and control program at Siteman. “But there’s a great deal we don’t know about the risks and benefits of these tests. Through the network, we can determine whether such tests can detect cancer early and save lives.”
Theranostics treatment couples cancer detection and destruction
Like the Greek chimera that is formed from parts of a lion, a goat and a serpent, the emerging science of theranostics is a powerful hybrid. The name itself is a fusion of therapy and diagnostics, and theranostic molecules are fusions as well, comprised of one part that finds and visualizes the tumor and another that destroys it. With the aid of this molecular chimera, scientists can locate, characterize, treat and monitor cancer all at once.
“Theranostics allows for truly personalized medicine in a way that hasn’t really been possible using other techniques or technologies,” said Daniel L. J. Thorek, PhD, an associate professor of radiology at WashU Medicine’s Mallinckrodt Institute of Radiology and of biomedical engineering at WashU’s McKelvey School of Engineering and co-leader of the Siteman Oncologic Imaging Program. “Traditionally, everyone with the same disease receives the same drug regimen. With theranostics, because we have the ability to image where the drug is going and how much is being taken up and retained in the tumor, we have a way to tailor the dosing and the schedule to each patient in a way that’s really unique.”
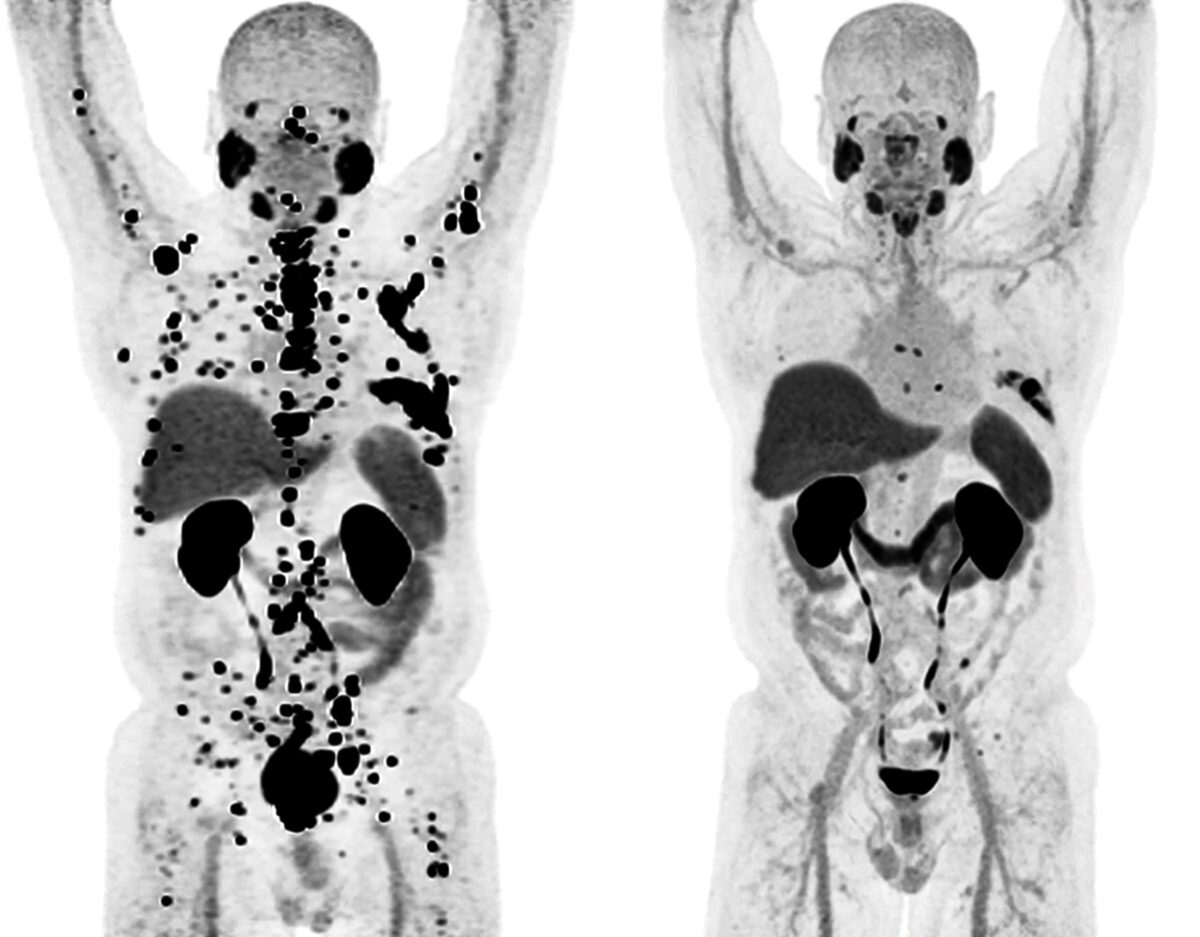
Thorek works on theranostic drugs that use alpha particle emitters, a kind of radiotherapy in drug form. These drugs are composed of a radioactive heavy metal attached via chemical compounds called chelators to a targeting molecule that homes in on the cancer. Thorek is working on novel chelators that can carry a larger number of heavy ions, thereby increasing the dose of radiation to the cancer and, ideally, destroying it more effectively. Studies in animals bearing human prostate cancers have been promising, and Thorek is now working toward a trial to determine whether the experimental drug behaves as expected in the human body.
“One thing that’s exciting about these drugs is how well tolerated they are,” Thorek added. “The side effects don’t seem to be as severe or as prolonged as with conventional chemotherapeutics. I think we’re about to see a widening of the scope of this field. The potential of theranostics to make better drugs for patients is enormous.”
Imaging reveals clues to tailored treatment decisions
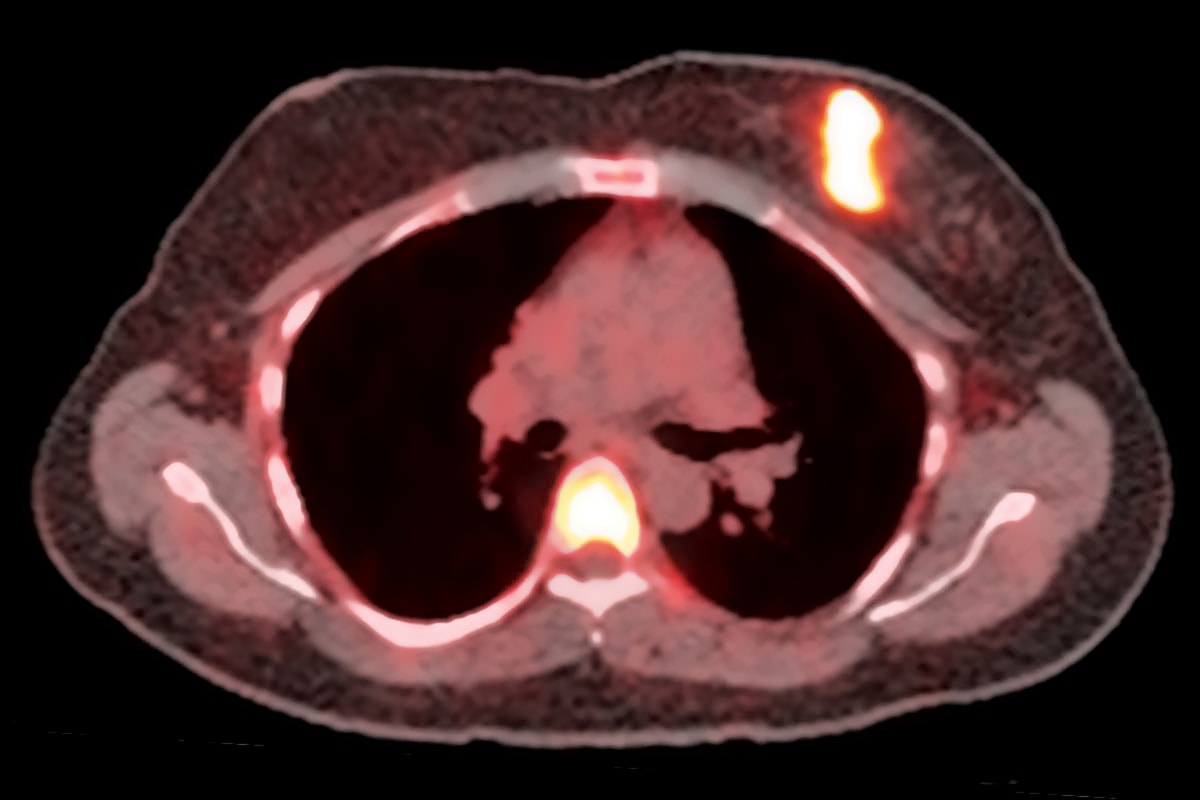
Cancer imaging is more than just detecting the cancer, according to Farrokh Dehdashti, MD, the Drs. Barry A. and Marilyn J. Siegel Professor of Radiology at WashU Medicine’s Mallinckrodt Institute of Radiology and co-leader of the Siteman Oncologic Imaging Program. As national leaders in cancer imaging, Dehdashti and other WashU Medicine researchers are pioneering advanced techniques and technologies that drive innovations in cancer care.
“These days, we can also characterize the cancer,” she explained. “We can see clues to how it is behaving, which is very important for knowing how to successfully manage and treat that cancer.”
For those who know how to see them, tumors carry molecular markings that reveal their strengths and weaknesses — which therapies they can resist and which they can’t. Dehdashti’s task is to make those markings visible so they can be read, interpreted and used to guide patient care.
For example, breast cancer is often treated with hormone therapy that targets estrogen receptors on cancer cells. Dehdashti and colleagues developed an imaging test that measures whether estrogen receptors on cancer cells are working properly, and used the results of that test to distinguish breast cancer patients who were likely to benefit from hormone therapy from those who were not. In a small phase 2 clinical trial published in 2021, she and colleagues showed that the cancers of all patients with working estrogen receptors remained stable or improved on hormone therapy — and progressed in all women with nonfunctional estrogen receptors. Now she is leading a larger, multicenter, phase 2 clinical trial to verify and expand on the initial study.
“If breast cancer in a patient is estrogen receptor-positive, doctors usually will recommend hormone therapy even though they know it will only work for slightly more than half the patients,” Dehdashti said. “When hormone therapy works, it’s typically quite effective, and it has relatively mild side effects, and that’s why oncologists and patients want to try it first. But if doctors know that hormone therapy is likely to be ineffective for a particular patient, they can start with another therapy that may work better.”
Bespoke cancer vaccines harness the power of the immune system
Cancer vaccines work by training the immune system to recognize cancerous cells and destroy them. The immune system has formidable weapons and can act with pinpoint precision, but it is also bewilderingly complex, and scientists at WashU Medicine are still learning how to harness its power.
Robert D. Schreiber, PhD, the Andrew M. and Jane M. Bursky Distinguished Professor, published a paper in 2001 demonstrating the immune system’s pivotal role in controlling cancer and laying the groundwork for the field of cancer immunotherapy. As co-leaders of the Siteman Tumor Immunology Program, he and William E. Gillanders, MD, the Mary Culver Professor of Surgery, are among the WashU Medicine scientists working to turn the promise of cancer vaccines into reality. Gillanders led an early breast cancer vaccine study that confirmed the potential power of vaccination and demonstrated the critical importance of choosing the right target. Schreiber and Gillanders have become leaders using genomics to identify neoantigens — mutant proteins unique to a patient’s tumor — and in using that information to develop personalized vaccines against various cancer types.
They’re now working to develop personalized neoantigen vaccines for patients with triple negative breast cancer and pancreatic cancer — both are aggressive and difficult to treat with standard therapies. Studies are underway to identify the best neoantigens and to understand the impact of combining vaccination with immunotherapies such as checkpoint inhibitors and immune modulators that developed broadly from Schreiber’s seminal studies.
“The immune system is immensely powerful, and we have never been able to control it as well as we can now,” said Schreiber, the director of the WashU Medicine Bursky Center for Human Immunology & Immunotherapy Programs and a WashU Medicine professor of pathology & immunology and of molecular microbiology. “The exciting thing is it’s just going to get better as we go along. So the big question is, how can we make cancer therapy more specific, more effective and safer? Vaccination used to be a pipe dream. Now we can determine the full genome of a tumor, so we have an opportunity for the first time of making a vaccine against the tumor cells specifically with no ill effects. We’re pushing back the boundaries, and it’s incredibly exciting.”
A new model to predict breast cancer
Middle-aged and older women are recommended to get mammograms every one to two years to screen for breast cancer. The widespread use of mammography has vastly increased the number of cancers found in the early stages of disease, when they are much more treatable. By some accounts, mammography screening programs have reduced deaths from breast cancer by up to 30%.
But if identifying breast cancer early is good, how much better would it be to identify people on track to develop cancer before tumors even form? This goal is what animates Shu “Joy” Jiang, PhD, a WashU Medicine associate professor of surgery in the Public Health Sciences Division.
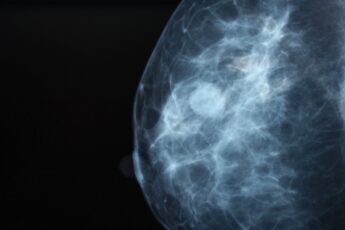
Jiang saw in repeated mammograms an untapped source of data on how breasts change in the years leading up to cancer diagnoses. In a 2023 study with Graham A. Colditz, MD, DrPH, the Niess-Gain Professor of Surgery; director of the Public Health Sciences Division in the Department of Surgery; and associate director of prevention and control at Siteman, and Debbie L. Bennett, MD, chief of the breast imaging section and an associate professor of radiology at WashU Medicine’s Mallinckrodt Institute of Radiology, Jiang used a mathematical model to monitor changes in breast density over the course of a decade in almost 1,000 women and found that the rate of change differed significantly between the nearly 300 women who were later diagnosed with cancer and those who were not.
In an ongoing study, she is using changes to breast texture over time to place women into one of five risk categories, ranging from very low to very high risk of developing cancer. Jiang and colleagues validated the model in three large datasets from the U.S., Canada and Sweden containing mammograms from more than 225,000 women. A diverse population including significant numbers of white, Black, Asian and Indigenous women in Canada was evaluated to ensure that the model works for women of all backgrounds.
Women who are at very high risk due to genetics are given the option of taking drugs such as tamoxifen to forestall disease. If Jiang’s model proves reliable, more women could become eligible for preventive treatment.
“I want to empower women to make their own decisions by giving them information about their risk,” Jiang said. “Many women already get regular mammograms, so the data is already being collected. We just need to use the data more effectively.”
Published in the Autumn 2024 issue


