
Kim Sebenoler, center, and her daughter, Sarah, along with other family members, are helping psychiatrist John Constantino, MD, understand the roots of autism. Kim carries, but does not express, a rare genetic mutation that may play a role in her twin boys’ autism.
Like many patients visiting a doctor’s office, Kim Sebenoler started out her appointment by heading to the nearest restroom to give a urine sample. But her visit to the lab of John Constantino, MD, director of the William Greenleaf Eliot Division of Child Psychiatry, was not a typical exam. The goal was not to measure proteins in her urine or check her overall wellness.
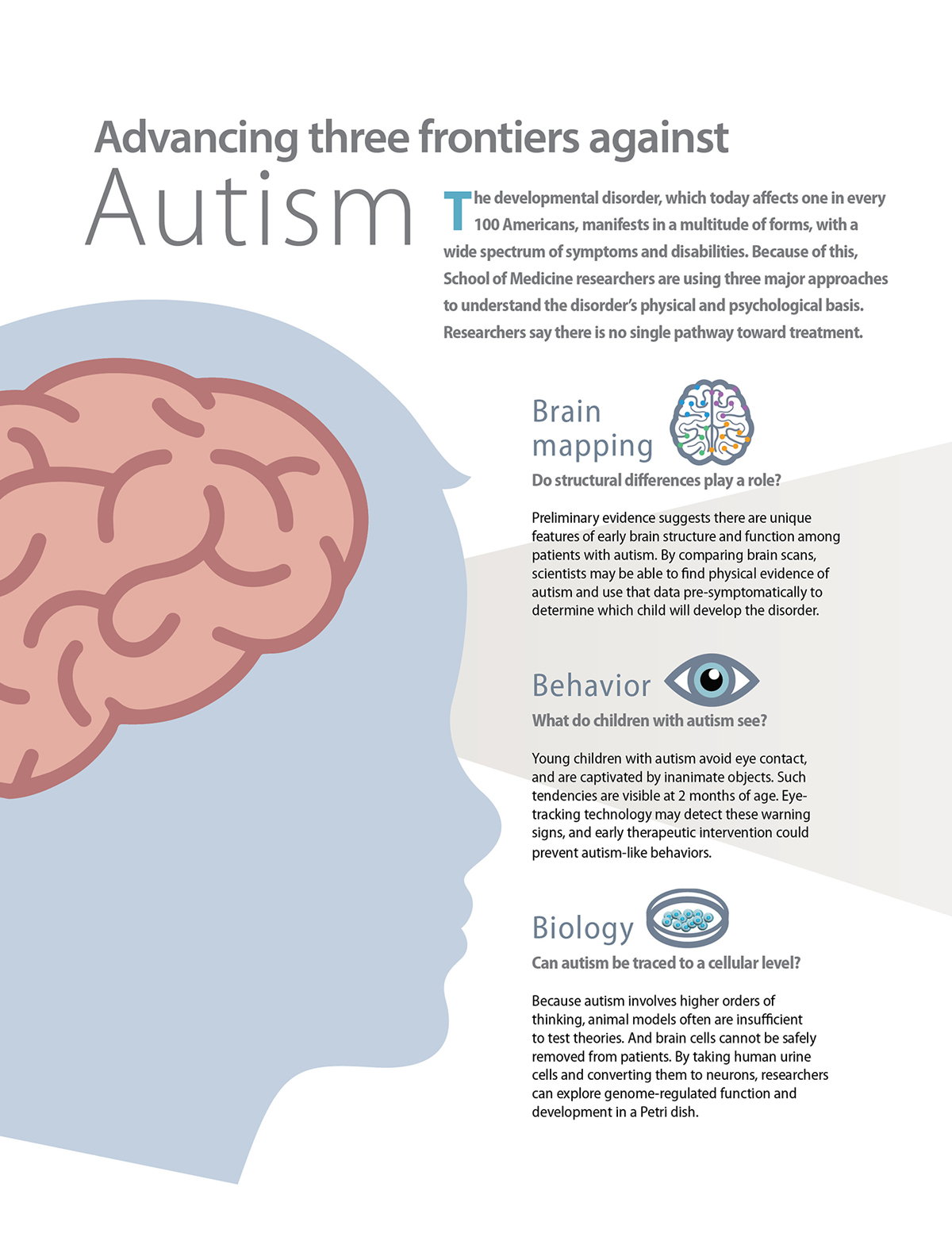
Instead, researchers took her urine cells to replicate human brain cell function in a Petri dish. The study is one of three major approaches School of Medicine researchers are using to unravel the physical and psychological underpinnings of autism. The unique, multifaceted effort — studying genes, brain activity patterns and behavior — is giving researchers and practitioners a better understanding of the disorder, which today affects one in every 100 Americans.
The cells are helping co-investigators Constantino and neuroscientist Azad Bonni, MD, PhD, explore how brain function changes in people with autism spectrum disorder (ASD). Both researchers are international leaders — Constantino in clinical autism studies and Bonni in advancing understanding of the underlying mechanisms of brain development.

The Kroll lab has figured out not only how to create neurons, but also to create neuron types that perform specific functions. These types — cortical excitatory neurons and inhibitory neurons — often are abnormal in patients with autism. This process includes a specific chemical treatment to generate these types of neurons in a Petri dish. In this case, populations of neurons are derived from the cells of the Sebenoler family. From left, Kristen Kroll, PhD, associate professor of developmental biology, and postdoctoral fellow Kesavan Meganathan, PhD.
Autism is most commonly characterized by repetitive behaviors and difficulty with communication and social interaction. It has a multitude of forms, and psychologists recognize a wide spectrum of skills, symptoms and disability.
“We are engaging people across disciplines — geneticists, molecular biologists, child psychologists, neuropsychologists and human brain imaging researchers,” said Bonni, the Edison Professor of Neuroscience and chair of the Department of Neuroscience. “There is no single pathway toward treatment or discovery. It’s absolutely the case that we need multifaceted approaches. Washington University is uniquely positioned to address these challenges.”
Researchers collected urine samples from several members of the Sebenoler family, including daughter Sarah, 18, and 16-year-old identical twin boys, Mark and Jack. Kim Sebenoler is not on the autism spectrum, and Sarah has not been formally diagnosed, but the twins were diagnosed at age 3.
How are neurons created?
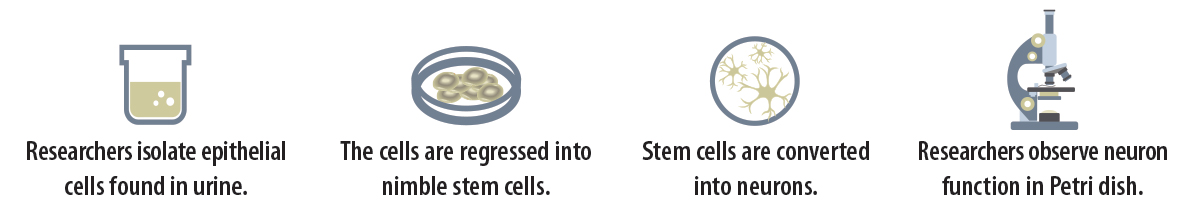
The researchers took the samples and separated solid material from liquid material. They isolated epithelial cells, a type of cell found in the lining of the kidneys that the body sheds all the time. From these, they produced induced pluripotent stem cells, which can become any type of cell in the body. Then, they reprogrammed the stem cells into neurons. This yielded a population of brain cells that could be studied, enabling side-by-side comparisions. Researchers are continuing to watch for variations in neuron viability and activity.
Later, the Sebenolers returned to the lab for brain imaging studies, so researchers can study activity in their entire brains, not just cells in a Petri dish.
The family has participated in studies on autism prevalence in twins, and Kim’s son Nicholas, 9, is part of a study examining the younger siblings of children with autism. Nicholas has participated since he was a baby, and is helping researchers study the earliest possible evidence for autism-related symptoms, which may be visible as soon as 2 months of age.
Ultimately, the researchers are trying to diagnose and treat autism as early as possible, aiming to develop treatments and possibly drugs that can halt or even reverse autism-related symptoms.
“Identifying genetic susceptibilities — and how they relate to the development of a human brain, and how genetic liabilities early in development could take a child off track — those are all of very high interest to us,” said Constantino, the Blanche F. Ittleson Professor of Psychiatry and Pediatrics and psychiatrist-in-chief at St. Louis Children’s Hospital.
He and Bonni say with early diagnosis and a suite of treatment options, doctors may be able to re-route children toward a developmentally typical track — rewiring their neurological trajectories, and eliminating or preventing autism-like behaviors.
Rates of autism diagnosis have skyrocketed in recent years, but scientists say this is due to improved diagnostic techniques, not necessarily an increase in people with the disorder.
Autism is highly heritable; a person with an autistic sibling is 20 times more likely than a person in the general population to also develop autism. Among identical twins, like Mark and Jack Sebenoler, if one twin has autism there is a 95 percent likelihood that the other twin will develop autism — evidence that suggests a strong genetic link. Most commonly, autism results from an accumulation of subtle genetic changes.
Female protective effect
With help from the Sebenolers and funding from the National Institutes of Health (NIH), Constantino and Bonni are trying to tease out genetic markers that might offer a protective effect against autism. Earlier, the researchers showed that autism is four times more likely to develop in boys than girls not because autism favors the male sex, but because most girls seem to be relatively protected. Capturing this protective effect — the effect of the extra X chromosome — could be a way to treat or reverse autism symptoms.
“Females carry the same genetic factors, but they don’t express them, which is a huge clue. We think that’s going on all the time in autism transmission,” Constantino said. “If we understood the biology of that, we could harness biology to treat the children who succumb to the genetic liability.”
In the Sebenolers, the research team has located a rare genetic mutation that is likely to have a significant role in causing the boys’ autism, and is carried (but not expressed) by their mother.
“We wanted to know, is there a way that we could identify, at the level of an individual cell, whether the neuron of a female carrying a mutation is behaving differently than a neuron of a male carrying this mutation?” he said. “If the difference is traceable to a cell, there is a better chance that pharmaceutical targets might be identified.”
In one study published last year, Constantino and colleagues at the University of California-San Francisco were unable to isolate a single gene that conferred protection to females. He is still looking, but noted that whatever biological defense is protecting females might not be obvious at the behavioral level. It might be more obvious at a cell- or tissue-based level, which is why the researchers have elected to differentiate populations of neurons derived from the cells of the family members themselves.
Alternatively, that protective effect might play out in a particular pattern of brain activity, which researchers can study using functional magnetic resonance imaging (fMRI). The Sebenolers, and many other patients, are taking part in studies that will look at all these factors.
Bonni said researchers are still trying to understand whether people on the spectrum share common traits. “It will be easier to develop therapies if we identify common mechanisms. On the other hand, assuming there are multiple different mechanisms, we will have to address those, and the approach that we’re taking with this family is the way to do it. That’s an example of laying the foundations for precision or personalized medicine. We have to consider both approaches as we move forward.”
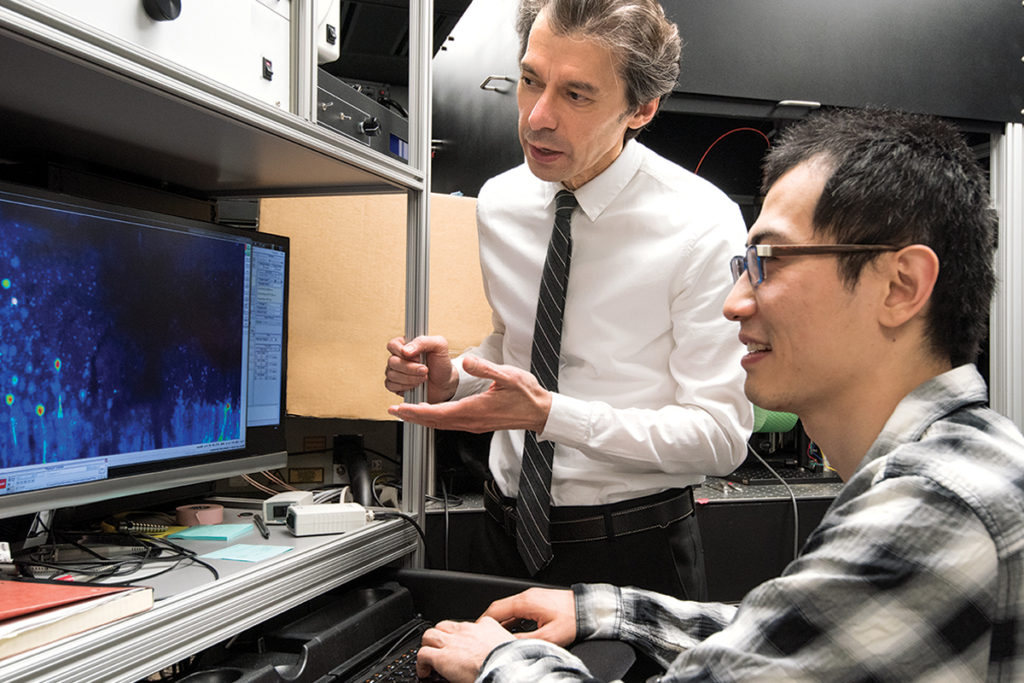
Over the past decade, Azad Bonni, MD, PhD, has contributed insights on how the brain is hardwired, and how neurons take shape and connect to each other. He also is determining how mutations in proteins implicated in autism cause problems. Here, Bonni (standing) confers with staff scientist Yue Yang, PhD.
Scientists have spent decades studying gene mutations that can lead to problems or disorders. Now they are increasingly focusing on genetic and environmental factors that might provide resilience to disorders, said Bradley Schlaggar, MD, PhD, the A. Ernest and Jane G. Stein Professor of Developmental Neurology, head of the Division of Pediatric Neurology, and neurologist-in-chief at St. Louis Children’s Hospital.
Schlaggar and Constantino co-direct Washington University’s 6-year-old Intellectual and Developmental Disabilities Research Center (IDDRC). This signature research effort seeks to find better prevention and treatment strategies for childhood developmental disabilities, including autism and the neurodevelopmental consequences of preterm birth.
The so-called “resistome” is more difficult to study, Schlaggar said, especially in formal clinical research, because there are so many competing and confounding factors; it’s difficult to control for every possible thing that could make someone more or less susceptible to autism.
“The pathway to treat the disease may not come from turning off or on the gene that is a problem, but recapitulating what the organism is doing to provide protection,” he said. “It’s not gratifying, though. It’s a strategy that doesn’t really lend itself to the usual scientific methods. It requires a big data approach. All the fundamental parameters of investigation are challenged by this strategy.”
Predicting outcomes
Just as genomics-based medicine is enabling personalized treatments, brain imaging is increasingly used not only to observe behavior and diagnose disorders, but to make predictions about a person’s possible outcomes.
Constantino, Schlaggar and their colleagues are using functional MRI and functional connectivity MRI to study brain architecture and understand how the brain’s physical attributes can affect a person’s psychological attributes. Schlaggar said studies on the brain’s organizational principles may help scientists understand what happens in atypical development.
He said most imaging studies look at the central tendencies of either patients and controls, or adults and children, and focus on the differences between them.
“That’s interesting, but it doesn’t get at the characteristics of individual patients. What you want to be able to do is use the data to make predictions about people,” he said. “We want to use data pre-symptomatically. Can we identify information from imaging that would allow us to predict which child is going on to that diagnosis?”
The research is still in early stages, but preliminary results suggest there are some types of brain activity scientists may be able to use to make predictions, Schlaggar said.
This level of analysis requires sophisticated software to crunch each person’s data. Network approaches are becoming increasingly useful tools for brain imaging research, and Schlaggar said he relies on Washington University’s multidisciplinary collaborations, such as those enabled by the IDDRC.
![]()
Eye tracking: The way young children explore the world with their eyes is under strict genetic control. When comparing the viewing habits of kids, patterns emerge. Bottom left, as illustrated by the crosshairs, normally developing children typically focus on people’s faces, particularly eyes and mouths. In atypical development, children fixate on inanimate objects. Identifying this lack of social engagement is a useful tool in early diagnosis.
“It used to be that you needed supercomputers to do this, but not now. The computational power we have just by linking our desktops here is amazing,” he said. “It’s been a really cool experience to see how these methods merge, and how they provide the kinds of insights we didn’t anticipate we would have access to when we started.”
Along with MRI imaging, Constantino is using eye tracking to catch autism-related behavior abnormalities in early development. Lack of eye contact is a distinguishing feature of autism. Several years ago, Constantino’s collaborators at Emory University in Atlanta published research demonstrating that infants with autism spend less time focusing on people’s eyes and faces, and more time focusing on objects. The eye-tracking differences were traceable to just 2 months of age.
Constantino wanted to look deeper to determine whether this difference was driven by the kind of genetic influences that characterize autism. He launched a study examining identical and non-identical infant twins and found that the way babies explore the world with their eyes is under stringent genetic control. He and his team also observed that some normal infants had as low a level of eye- and mouth-looking as the children with autism in the prior study.
“There has to be some other factor that combines with visual disengagement, to produce the autistic syndrome,” he said. “This gives us another opportunity to learn how susceptibilities to autism (like low levels of eye-looking) might be weathered and overcome. These are windows of observation on how various components of early human development collide, interact with or compensate for one another.”
Currently, the earliest possible diagnosis for autism is around 1 year old. Intervention programs, including behavior analysis, speech and language instruction, and occupational therapy, typically don’t start until age 2. Scientists suspect that very early interventions might improve the chances of redirecting a child’s development.
“These three frontiers — gene discovery, brain imaging, and development — all feed each other,” Constantino said. “We need to get our arms around as many factors as we can. We are thinking about all possible places where disparate genetic pathways to autism converge.”
Kim Sebenoler said she hopes her family can help researchers find those convergences. Along with participating in several studies, her children are in behavioral therapy programs and visit Constantino for psychiatric appointments on a regular basis.
“I like the spin that Dr. Constantino puts on it, which is, ‘Let’s see how far you can get.’ He sees the potential that’s in every child — that there’s not limitations,” she said. “These studies hopefully will end up in good results. So I’m very hopeful.”

Published in the Winter 2016-17 issue


