As the COVID-19 pandemic began to take shape in the first weeks of 2020, viral immunologist Michael S. Diamond, MD, PhD, and immunologist Ali H. Ellebedy, PhD, dove into the global race to develop vaccines and therapeutics against COVID-19. Years of training and experience had prepared each for just such a crisis.
The two quickly pivoted to studying how SARS-CoV-2, the virus that causes COVID-19, infects people and how the immune system fights back. Ellebedy investigated what a protective immune response looks like and whether natural infection and vaccination effectively induce such a response. Diamond developed a mouse model and with colleagues at Washington University created two vaccine candidates, one an inhalable nasal vaccine that is in advanced clinical trials in India.
Here, they reflect on the international effort to develop COVID-19 vaccines, the state of vaccine science, and how the field has changed under the extraordinary pressures — and opportunities — of a once-in-a-lifetime pandemic.
2020 started with vague reports that a new member of the coronavirus family was making people sick, and ended with health-care workers rolling up their sleeves to get their shots. Designing, testing and authorizing a vaccine against a novel virus — in under a year — was an extraordinary triumph of modern science. How did it happen?
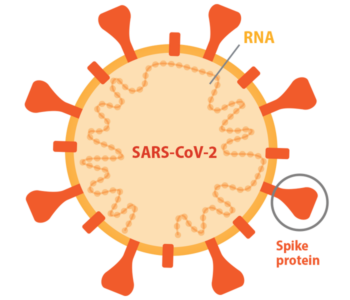
Diamond: We weren’t starting from scratch. There already was a body of research on the related coronaviruses SARS-CoV-1 (the original SARS) and MERS (the Middle Eastern Respiratory Syndrome virus). There were vaccines for SARS-CoV-1 in animal testing and vaccines for MERS-CoV in animal testing and in early-phase clinical trials. These vaccines all targeted the same viral protein: spike, which the viruses use to get inside cells. And they worked in animals. So that told us, right away, that spike was probably a good target for a SARS-CoV-2 vaccine.

Another important advantage is that we knew the structure of the spike protein. Sometimes, a virus emerges from a family that we just don’t have a lot of information about. Here, we had a very high-resolution structure of spike from related coronaviruses. We also knew how to mutate the spike in such a way to produce the best antibodies. Scientists quickly figured out that SARS-CoV-2 uses the same human receptor to get into cells as SARS-CoV-1; MERS, in contrast, uses a different one. This allowed us to take all of that information we already had on how antibodies block SARS-CoV-1 binding to the receptor and translate that to SARS-CoV-2. And finally, of course, the messenger ribonucleic acid (mRNA) vaccine platform already was under development.
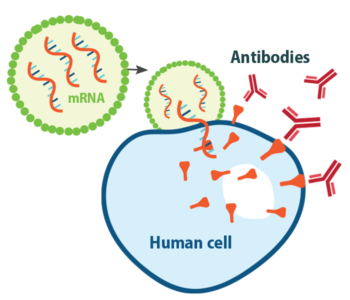
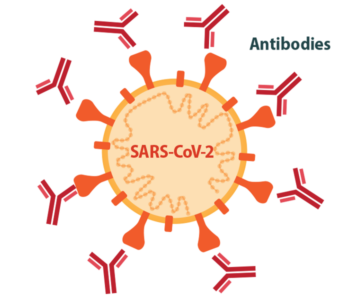
Ellebedy: We knew that most successful vaccines for viral infections work by eliciting high levels of neutralizing antibodies, so that’s where we started. Then, we learned from early studies of people who were infected with SARS-CoV-2 that many of them developed potently neutralizing antibodies upon natural infection. That was a very good sign. If natural infection induces a good immune response, then all you have to do is mimic that natural response. Those early studies told us that it should be possible to make a successful vaccine, and what that successful vaccine should target.
A pandemic is a catastrophe, of course, but it’s also an opportunity for people in your field. What have vaccine scientists learned from this pandemic?
Ellebedy: The main thing we learned is that, in the past, we took far too long to make new vaccines.
Diamond: The shortest one before was measles, at four years. Most vaccines have taken the better part of a decade, and some were 20, 30 years in the making. We learned by use of new vaccine platforms that as soon as we had the genetic sequence of the virus we could make a vaccine as long as we understood some of its biology. Before, we didn’t think developing a vaccine for humans in this time frame was possible.
Ellebedy: Ten years, 15 years from now, when we look back at this time, I think we’ll see that one of the silver linings of this pandemic is that it provided the opportunity for the mRNA platform to be utilized on a global scale. I wasn’t here in St. Louis when the work was done on the Zika mRNA vaccine in Mike’s lab, but I saw the preclinical data and it was very impressive. But luckily we did not need to deploy that vaccine globally. A major advantage of the mRNA platform is that it can be adapted quickly. I think it’s definitely going to be used against other viruses like influenza, where you really need the ability to make changes quickly. And the ability to include multiple mRNAs against multiple strains in one shot is certainly a plus.

Diamond: And also, we’ve learned a lot about B cell responses, which are the group of cell types that produce antibodies. We knew some of it from flu and other viruses before. But during this pandemic we’ve learned about how B cell responses occur in individuals infected or vaccinated for the first time, individuals infected after vaccination, individuals vaccinated after infection. We’ve learned about the B cell response to viral variants. Those are all important points that will translate to other pathogens as well.
A lot of people hoped that vaccines would be a silver bullet and stop the COVID-19 pandemic in its tracks. They’ve helped, but the pandemic is still going. Why?
Ellebedy: This pandemic revealed the huge disparity between developed and developing countries in terms of vaccine access. After this pandemic, as a global population, we really need to take a look at how we can fix that because we’ve learned that you cannot just vaccinate your population and expect that things will be fine. A variant can come from an under-vaccinated area where the virus is circulating and basically nullify all your efforts to control the virus. We should care about the pandemic situation in places where vaccination coverage remains poor like Egypt, India, South Africa and many others.
Diamond: Part of that is financial, but part of that is there just wasn’t the infrastructure to deliver these high-efficacy vaccines. There were worldwide cold-chain issues, meaning that it was hard to ensure that the vaccines stayed at the proper temperature from the time they left the factory to the time they were injected. Vaccines will not end a pandemic alone; you need other public-health measures, including testing. Ali mentioned earlier the situation in Egypt, where people don’t bother to try to get tested because it’s not reliable or they can’t get tests or the test doesn’t come back in time. There are many places in the world where testing is still a problem nearly two years into this pandemic. That’s another huge inequity that we need to solve.
Has the global race to develop vaccines for SARS-CoV-2 revealed any blind spots or weaknesses in the field of vaccine science?
Ellebedy: The vaccines that were created were amazing, but we probably needed a mucosal vaccine that can elicit a strong immune response at mucosal surfaces. It is almost impossible to control transmission of a respiratory virus without having a really strong, very solid immune response at the site of entry: the nose. The problem is that we don’t really have many successful examples of good mucosal vaccines. Historically, most vaccines are injected, and we measure their immunogenicity by assessing antibody levels in the blood. Antibodies in the blood prevent the virus from spreading from the upper airways through the blood to other parts of the body, like the lungs, where it can cause more severe disease. Early COVID-19 vaccines do induce a strong antibody response in the blood, which is why they have been very effective at preventing severe disease, hospitalization and death. But they don’t do a great job of preventing infection, and that’s because we don’t really know much about antibody responses in the nose. We don’t know how to tell if we have enough antibody there for protection or how long those antibodies will last.

Diamond: The first goal was to get people out of the hospitals, stop people from dying, and get back to some sense of normalcy and any vaccine that did that reasonably well was a success. The fact that the mRNA vaccines were 90% to 95% efficacious was beyond our wildest dreams. But we designed the vaccines based on assumptions about human behavior that turned out not to be true. We had an expectation that if we had an effective vaccine, everybody would get it. Like last century, everybody got the smallpox vaccine. That’s how we got rid of smallpox. Same for polio. But here, in the U.S., and especially in Missouri, you have swaths of the population that remain unvaccinated and therefore vulnerable to infection. Where you have so many people that remain susceptible, a regular vaccine that prevents severe disease will not break the cycle of transmission. You need to have a vaccine that stops transmission. That’s the only solution to it. And that would be a mucosal vaccine … that people take.
Ellebedy: Another thing we learned during this pandemic was that speed matters. Not only do you need to be fast in developing a vaccine, but also fast in advancing it through clinical testing. Academic research centers like this one have historically not been set up to rapidly move a vaccine candidate from the lab to clinical trials.
Diamond: Ali and I, along with Rachel Presti, the director of the Infectious Disease Clinical Research Unit, have established a Vaccine Research Center here at WashU. The plan is to take advantage of our outstanding community here to develop a pipeline of vaccine discovery — starting from the most basic aspects of vaccine science, going through small animal studies and then into phase 1 clinical trials through the center. And then we’d help advance the candidates that are the most successful through later-stage clinical trials and, ultimately, ideally, get FDA approval. The center will be not just for viruses, but also for bacteria, parasites, fungi, any pathogen that could be targeted with a vaccine, especially those for which there are currently no good vaccines or therapies. We have a huge amount of expertise at WashU in microbiology, immunology, infectious diseases, as well as other relevant areas such as pediatrics, emergency medicine and nuclear medicine, that we can tap into to design vaccines, evaluate them and get them into people.
Published in the Winter 2021-22 issue