During his freshman year of college, Brian Phillips came home one day to find his parents unexpectedly already there. With tears in his eyes, his dad, a former Marine, put his hand on his son’s shoulder and asked him to sit down. “I’ll never forget it,” Phillips said. “I’d never seen my dad cry before.”
The test results were in. At age 19, Brian had just been diagnosed with multiple sclerosis.
He had known something wasn’t quite right for a while. Tennis had always come easy to him. But when he challenged a friend to a game, he played so badly that he completely missed many of the shots. Following some tests, including an MRI brain scan, he received the diagnosis.
A multifaceted disease
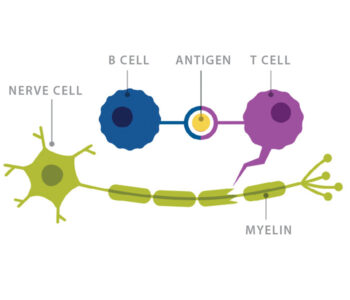
Multiple sclerosis, or MS, is a chronic, often disabling disease in which the immune system attacks the myelin, the fatty substance that insulates axon nerve fibers and makes up the brain’s white matter.
The multiple forms of MS present with variable symptoms depending on the individual. These may include impaired motor function, lack of coordination, blindness, neuropathic pain, bladder and bowel issues, extreme fatigue and more.

Further from the equator, disease rates are higher. This suggests a link to a lack of vitamin D, which the skin makes in response to sunlight, but this has yet to be proven as a definitive cause.
In the U.S., over 600,000 people live with the disease — 70% of whom are women. The age at diagnosis ranges from 20 to 60 years old.
After encountering three colleagues and many patients with MS during her medical training, neurologist Anne Cross, MD, made it her life’s work to study and treat patients with the disease. “As a resident, I saw a lot of MS patients. They were predominantly young women my age and there was really nothing we could do for them,” she said.
Expanding the arsenal
With few drugs available, Cross, now director of the John L. Trotter MS Center at Washington University, decided she wanted to help discover treatments through research. Even though she had no research experience, she was accepted into a fellowship working with two highly respected MS researchers at the National Institutes of Health: Dale McFarlin, MD, and Henry McFarland, MD.
“They really took a chance on me. I had not done any research before that. I didn’t even know how to hold a pipette,” said Cross, the Manny and Rosalyn Rosenthal–Dr. John Trotter MS Chair in Neuroimmunology. The work she did there was similar in scope to a PhD program, she added.
Cross completed two more fellowships before being recruited to the School of Medicine with her husband, DeWitte Cross III, MD, a professor of radiology and of neurosurgery. They moved to St. Louis with their young son (now a third-year neurosurgery resident) and daughter.
It was here she met John Trotter, MD, one of the first neuroimmunologists, thanks to his unique training at the time in both neurology and immunology. Trotter, who died unexpectedly in 2001, cared for half the patients with MS in St. Louis at the time of his death.
B vs. T cells
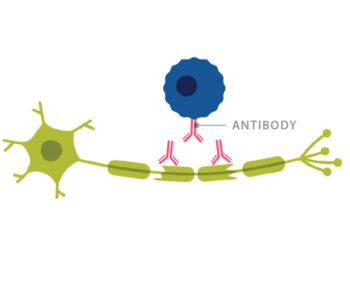
Trotter ran a lab at Washington University and Barnes-Jewish Hospital that focused on identifying oligoclonal bands of antibodies in spinal fluid. Oligoclonal bands are present in over 90% of people with MS, making them valuable in diagnosing the disease. Increased antibodies in spinal fluid also implicate B cells in the disease process, as B cells make antibodies.
Trotter asked Cross to co-author a textbook chapter summarizing the literature on B cells and antibodies in MS. This prompted Cross to think about how B cells might be involved with MS.
Credit: Genentech, a member of the Roche Group
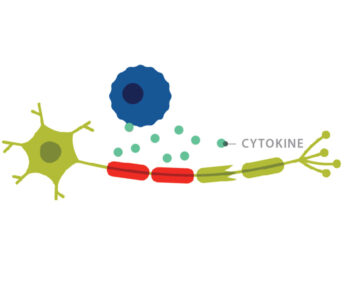
At the time, T cells were believed to be the main player in MS. Researchers already knew that it took T cells, not B cells, to generate the animal model of the disease, Experimental Autoimmune Encephalomyelitis (EAE). To activate this disease process in a mouse, researchers had to immunize the animal with myelin oligodendrocyte glycoprotein (MOG). This would induce autoimmune T cells to lead the attack on the mouse’s own myelin.
Additionally, T cells are more abundant than B cells in areas of demyelination in MS tissues. “Everybody was interested in T cells,” Cross said. “So, I thought: ‘Let’s look at B cells.’”
To better understand the role of B cells in MS, Cross and her postdoctoral fellow at the time, Jeri Lyons, PhD, (now at the University of Wisconsin-Milwaukee) found mice that had been genetically altered to lack B cells. These mice would allow the researchers to test a hypothesis: that without B cells, the mice would be unable to develop EAE.
When they tried to induce an autoimmune response in the B-cell deficient mice by immunizing with MOG, the mice remained healthy. But when they performed the same MOG immunization in mice with B cells, those animals developed EAE. This clearly implicated B cells as critical cells for EAE development in mice.
“It was one of the most dramatic experiments I’ve done,” Cross said.
Using these exciting results, Cross and her team decided to test the idea that B cells were contributing to MS.
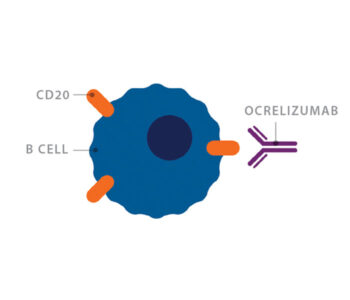
Rituximab, a drug that depleted B cells, had recently been approved by the FDA for non-Hodgkin’s lymphoma, a B cell cancer. Cross convinced Genentech, the company that made rituximab, to allow her to use the drug for the first time in a clinical trial looking at patients with MS. Her results were encouraging. MRI activity in the patients went down by 88% after treatment with rituximab.
Rituximab is a monoclonal antibody that targets the molecule CD20 on the surface of B cells. CD20 is only found on B cells, so the drug can selectively eliminate B cells, while other cells remain unaffected.
Genentech subsequently developed ocrelizumab, a similar, second-generation version of the drug rituximab, for use in people with MS.
The most common form of MS, relapsing remitting MS, is characterized by debilitating attacks on the central nervous system, alternating with periods of remission. Primary progressive MS is less common — affecting only about 10 to 15% of patients — and is characterized by a progressive worsening of neurologic impairment with no remission. Many with relapsing remitting MS will develop secondary progressive MS, which presents initially as relapsing and remitting, but then becomes progressive.
Cross was Washington University’s site lead in a study looking at ocrelizumab’s effects on patients with primary progressive MS. The study showed that those taking ocrelizumab had a slower progression of disability compared with a placebo. In 2017, ocrelizumab became the first FDA-approved drug for this form of MS.
Expanding the field
“B-cell therapy in MS is the fastest growing therapy we have,” said Robert Naismith, MD, professor of neurology and director of the Clinical Trials Program at the John L. Trotter MS Center. Naismith analyzed MRI scans of patients in the rituximab trial, serving as the “blinded” investigator, meaning he did not know which patients received the drug.
Imaging advances have dramatically changed the field of MS, as MRI scans now can enable physicians to diagnose the disease sooner. Cross also has been actively involved in researching new imaging techniques to advance the study of MS.
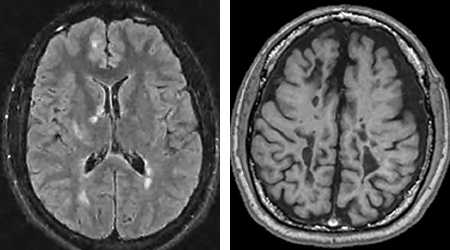
Typically, MS is diagnosed through MRI scans of the brain and spinal cord, which can detect lesions in the white matter, indicating loss of myelin. If the lesion is “active,” meaning there is inflammation in that area, it can pick up a contrast or dye called gadolinium that appears as a bright spot on an MRI. As the disease progresses, lesions may appear as “black holes,” which indicate more severe damage to the underlying axons or nerve fibers.
Around the time the rituximab study was starting, Cross formed a collaboration with Sheng-Kwei (Victor) Song, PhD, professor of radiology. They became among the very first to use a novel MRI scan called Diffusion Tensor Imaging, which measures the diffusion of water through tissue, to better image the demyelination process in MS.
Naismith has since expanded his role, now overseeing more than 12 MS clinical trials that look at everything from new imaging techniques and drug therapies to the effects of intermittent fasting on MS.
No drugs are risk-free, and those available for the milder forms of the disease, while less risky, are often not very effective. For patients who have not had luck with the weaker treatments, Naismith said drugs like rituximab and ocrelizumab have still shown relatively few side effects and are convenient since they are given intravenously twice a year.
Naismith and Cross are investigating other MS therapies, including a B-cell depleting drug that can be taken subcutaneously, as well as oral medications. They also are planning studies with more personalized therapies that would modify a patient’s own immune cells to fight the disease. He added the next frontier of MS therapy involves researching drugs that may help with remyelination and possibly even axon regeneration.
This year, Cross received the prestigious John Jay Dystel Prize for MS Research, an award that recognizes outstanding contributions to research in the understanding, treatment or prevention of MS. She won this primarily for her role in demonstrating the importance of B cells in MS.
“It is the prize in MS recognizing career achievement,” said Jack Antel, MD, professor of neurology and neurosurgery at McGill University and past president of the International Society of Neuroimmunology.
“Anne is recognized as a very distinguished, caring and competent physician. She is a true physician-scientist.” He added that she has a knack for translating therapies from the experimental phase to the clinic and vice versa. “If she gives a clinical opinion, you respect it. If she presents research, you respect it. If she gives advice on how an organization should run, you respect it.”
Cross said that when she first started in the field, there were few to no viable MS treatments. “Now we have so many medications it makes it hard to choose,” she said. “We have MS treatments that don’t just treat the symptoms, they actually slow the disease.”
But physicians like Cross still struggle with determining which patient should receive which drug, since it is not yet clear who will develop a more aggressive form of MS that might need a more aggressive, but riskier, drug and who will not. There are some people with MS who were diagnosed early, began therapy and decades later you wouldn’t even know they had the disease, Cross said. “While with others, we can try almost every drug available yet they still go downhill,” she added.
Cross’ patient, Brian Phillips, said he feels fortunate and tries to maintain a positive attitude. Shortly after his diagnosis in 1998, he had a Gaelic saying tattooed on his ankle: “To the valiant heart, nothing is impossible.”
While he is legally blind and cannot drive, he walks unassisted and generally goes about his daily life, which includes working and spending time with his wife and two children. Over the years, he has been on a number of MS drugs and is currently taking rituximab, which he started three years ago. “I haven’t had a relapse in 12 years,” he said. “I feel like I’m in a good place.”
Gaia Remerowski is a senior content strategist in Medical Public Affairs.
Published in the Winter 2019-20 issue