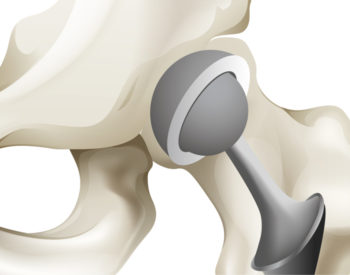
Replacing a human joint is common, artificial and far from permanent. The procedure involves cutting into the body to remove living bone and replacing it with metal and plastic. As these parts typically last 15 to 20 years, a second — riskier — replacement surgery sometimes is necessary.
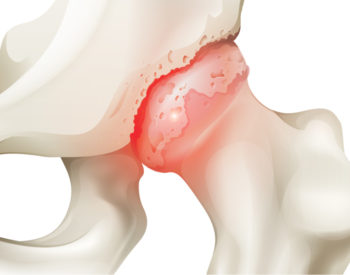
However, for a million Americans a year in agony with knee or hip arthritis, such implants are welcome. Because once cartilage, the tissue lining and lubricating joints, wears away, the patient is left with bone-on-bone pain, swelling, stiffness and disability.
School of Medicine researchers are working on a radically different way to help people with arthritis: creating living joint replacements from the patient’s own cells and then programming those cells to fight an arthritis recurrence.
Farshid Guilak, PhD, and collaborators have successfully grown part of a living human hip joint in the lab and now are testing the methodology in animals. The development ultimately could transform how arthritis and some orthopedic conditions are treated. Moreover, aspects of the research have implications for a host of other diseases.
For Guilak, it all began with envisioning cells as machines.
“My background is in engineering, but I’ve always worked on cells,” said Guilak, a professor of orthopaedic surgery, of developmental biology and of biomedical engineering. “Cells are the basis of life, but they also are like little machines. They have motors. They store things like computers do, and they can generate forces and crawl around and pull on things.
“They’re little machines that happen to be alive, and we thought, ‘Why not treat them like machines and reprogram them to become what we want them to be?’ And our first thought was to make cells into things that can treat diseases automatically.”
Guilak, also the co-director of the Washington University Center of Regenerative Medicine and director of research for Shriners Hospitals for Children-St. Louis, is working in a field known as synthetic biology. Combining aspects of engineering and biology, researchers design and build new cellular components to do something different from what they do in nature.
Guilak manufactures cells programmed to combat arthritis. His lab is one of the few labs in the world doing this type of work. Until now, most synthetic biology research has been conducted in bacteria, which are easier to modify than cells.
“We began to work in mammalian cells, and it was difficult when we first started, but we got very lucky because as we were launching this research, the technique called CRISPR/Cas9 came on the scene, allowing us to quickly edit the genes in these cells,” Guilak said.
CRISPR/Cas9 technology enables researchers to alter DNA sequences and modify gene function.
Regrowing cartilage
Starting this process requires the manufacture of a basic building material: human cartilage. First, Guilak’s laboratory acquires stem cells from skin or subcutaneous body fat via liposuction. Then, the researchers treat those stem cells with substances that convert them into particular cell types. “If you pull those cells out of the body and give them a very defined set of proteins and genes, they’ll start to make cartilage or bone or even muscle,” Guilak said.
In this case, researchers treat the stem cells so that they grow into cartilage, one of the principal tissues damaged by arthritis.

But Guilak’s team was not content to use stem cells of ordinary “intelligence;” the researchers wanted to make them smarter. Using the gene-editing tool CRISPR/Cas9, the researchers excised a gene in the cells associated with inducing inflammation and replaced it with a gene that dampens it. They dubbed these cells as “SMART” (Stem cells Modified for Autonomous Regenerative Therapy).
Inflammation is associated with all forms of arthritis and triggers much of its pain and discomfort. Osteoarthritis is a degenerative, inflammatory condition caused by wear and tear on specific joints. Rheumatoid arthritis, an autoimmune disorder, occurs when the immune system attacks tissues in joints as if they were foreign invaders.
Through an analysis of arthritic cartilage, Guilak’s former student Jonathan Brunger, PhD, found that a particular gene, activated by inflammation, sparks further inflammation in response to a molecule called tumor necrosis factor-alpha (TNF-α). This molecule is primarily responsible for the inflammatory response in rheumatoid arthritis. Several available drugs block the TNF pathway to treat arthritis.
“These drugs work in about half of all patients,” Guilak said, “but they’re given at high doses continuously, even though the disease waxes and wanes. What you really want is something that’s only given when there is a flare in the disease.”
Such medications reduce inflammation, but also carry side effects; they can suppress the immune system, affecting the body’s ability to fight infection.
The gene that Guilak and Brunger inserted into the cartilage cells is activated only during bouts of arthritis inflammation, targeting the same pathway as the drugs Enbrel, Humira and Remicade. “We can control how a cell reacts to the external signals it receives,” Guilak said. “We can use CRISPR/Cas9 to make new logic circuits inside a cell.
“In this case, it’s called a closed feedback loop because the cell has the capacity to shut down something that’s happening in its environment. Instead of encountering an inflammation signal and amplifying it to get bigger and bigger, which is what happens in arthritis, now when these SMART cartilage cells see this inflammatory molecule, they make the drug inhibitor to block it.”
Arthritis and beyond
Guilak’s team is working to combat the differing types of arthritis. Solutions range from a living joint replacement made with SMART cells for localized osteoarthritis to a possible implant or vaccine for the systemic disorder rheumatoid arthritis. Encased SMART cells could be placed under the skin the same way Norplant birth-control devices deliver hormones to prevent pregnancy for several months or years at a time. Likewise, because this strategy heightens immune response, a vaccine might be an effective biological treatment.
In all of these scenarios, it’s possible, Guilak, said, that a patient could turn the gene on and off as needed by taking a pill. The idea is to protect the body both from inflammation and excessive medication.
One of Guilak’s principal collaborators is Christine T. Pham, MD, a professor of medicine who treats patients with rheumatoid arthritis. Her lab has developed several mouse models of the disease, allowing researchers to test different treatment methods.

The researchers also have set their sights well beyond arthritis. Already, they are thinking about cellular feedback loops to deliver substances to fight other disorders, for example, cells that could be engineered to release insulin in response to high blood glucose.
For now, however, the primary strategy involves creating living cartilage from a person’s own stem cells to ease pain and keep the disorder at bay.
A living prosthesis
Removing a worn-out artificial prosthetic can destroy the attached bone and put patients at risk for infection. Because of this, doctors are reluctant to perform artificial joint replacement in patients under age 50, leaving children and young adults with few options. Rising rates of obesity and arthritis mean that more people in their 40s are needing hip replacements.
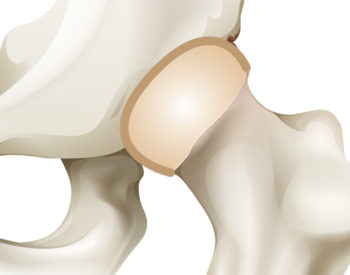
SMART cartilage fashioned in the form of a living joint potentially could outlast those made of metal or plastic. Researchers also believe there’s a lower risk of rejection because the cartilage cells are taken directly from the patient.
Stem cell treatments for osteoarthritis began as early as 2008. Mostly, doctors have injected stem cells directly into affected areas, hoping the cells would stay in place and turn into cartilage. But this method has proven unsuccessful, as the cells tend to float away within a couple of weeks.
Guilak has spent years working on a method to keep the new cartilage cells in the joint. Before coming to Washington University, he and his Duke University colleagues developed a way to weave 600 bio-compatible fibers approved by the U.S. Food and Drug Administration into a porous, high-performance fabric scaffold.
Using MRI images, the researchers can mold the fabric into the precise shape of a patient’s joint and seed it with stem cells. “Over a six-week period, the cells go inside the fabric, start growing, and are given chemicals that turn them into cartilage cells. The fabric eventually melts away, leaving a hip made out of your own cells,” Guilak said. “In the future, this technique could be used for any joints.”
The weaving pattern makes the implant strong enough to withstand loads up to 10 times a patient’s body weight — the standard force during exercise.
Guilak and the other researchers patented the weaving technique and formed a start-up company, Cytex Therapeutics Inc.
His laboratory has demonstrated the technique works in human cells in culture, and he’s conducting animal studies that look very promising. As a result, Guilak has won the Arthritis Foundation’s highest award, and the Proceedings of the National Academy of Sciences has published the findings. If those trials remain successful, human safety studies could begin in three to five years.
Despite worldwide media attention on the research, Guilak said he is most motivated by his daily walk to work. “Part of our lab is at Shriners Hospitals for Children,” he said. “And we see so many kids come through with difficult problems. They have a whole lifetime ahead, but almost everything we do for them is temporary, or it’s something like a metal-and-plastic implant, which isn’t a great treatment for a child who will outlast those implants several times over.
“Seeing those kids is part of what drives us to find better solutions. Our concept is that we can make cells that are like tiny computers that sense what’s causing problems and act accordingly.
“We’re confident we’re on the right track.”
Published in the Winter 2017-18 issue